OSMOTRON WFI
The shortest and most cost-effective way from potable water to WFI
- WFI production with the membrane process in line with Ph. Eur. und USP
- Triple membrane barrier for maximum quality and safety
- SEPTRON WFI electrodeionization including final ultrafiltration with MWCO in line with USP
- Simple and environmentally friendly with no salt, no handling issues, and no waste water problems
- Up to 15 m³/h WFI on a compact frame, fully pretested
Three times as impressive
This product never fails to impress, even in the smallest spaces.
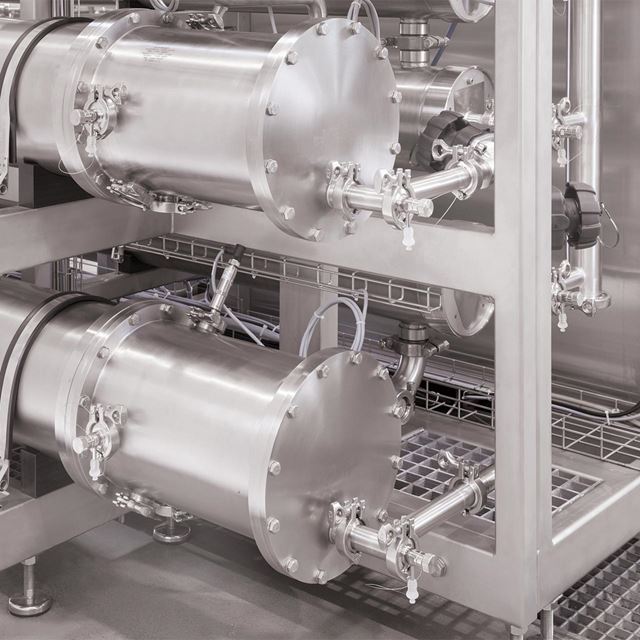
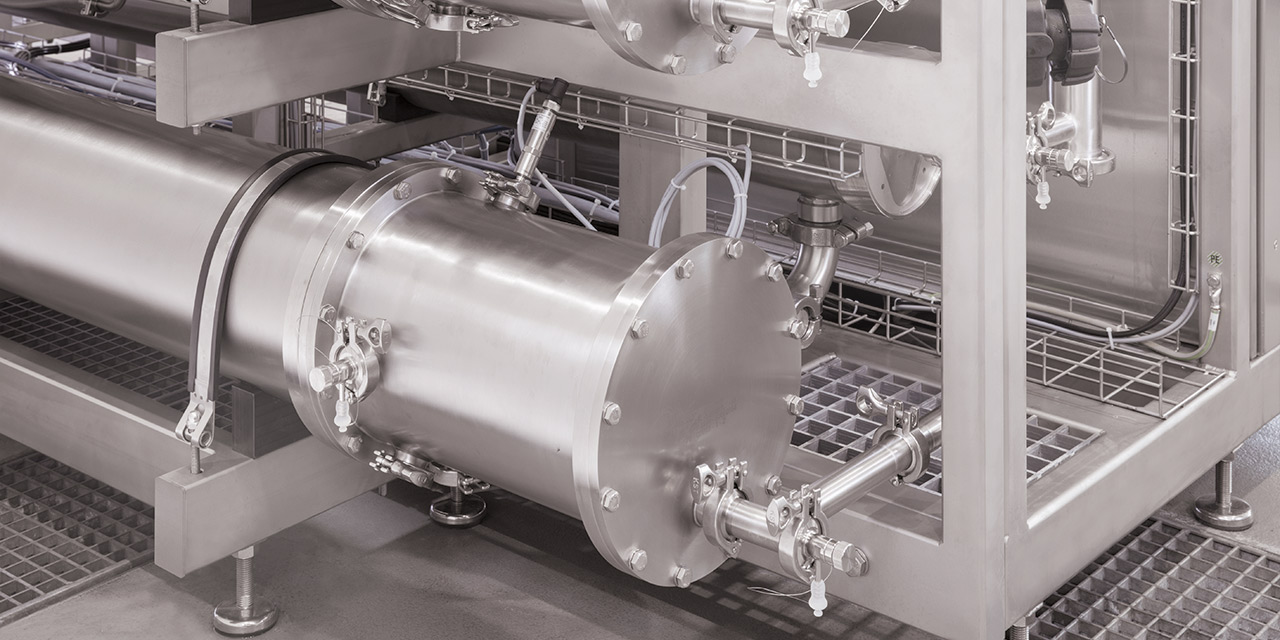






A milestone
Membrane process for the best WFI.
The European Pharmacopoeia only prescribed distillation as a method for producing WFI for a very long time. Even back then BWT had shown in practice with many customers that the OSMOTRON can easily adhere to the microbiological and chemical thresholds set out for WFI. As it became clear that the WFI monograph of the Ph. Eur. would be changed, one thing became clear for the whole BWT team from the very beginning:
If the focus is on quality, we don't compromise!
As a result, the OSMOTRON WFI was totally revamped. The requirements of the pharmacopoeia, the design of annex 1 and the Q&A of the European Medicines Agency were the foundation. The system was developed in line with quality-by-design principles using a risk-based approach. Comprehensive long-term tests were carried out in cooperation with an independent university. In this case, the system's performance is verified with valid data.
Downloads
| Membrane-based WFI in Best Quality | PDF, 1502 Kb | Download | |
| AQU@ Service - Critical Utilities in the best hands | PDF, 3 Mb | Download |
Unexpected error. Please try to reload the page














